Abstract
Introduction: Therapy-related myeloid neoplasm (t-MN) includes acute myeloid leukemia (AML), myelodysplastic syndrome (MDS), and myelodysplastic/myeloproliferative neoplasms (MDS/MPN) that occur as a complication of DNA-damaging therapies. The World Health Organization recommends considering t-MN as a single entity. Whether t-MDS and t-AML have distinct characteristics and outcomes is not known. The aim of this study was to compare clinicopathological characteristics and outcomes of t-MDS and t-AML.
Methods: All patients diagnosed with t-MN based on the World Health Organization criteria were identified. Bone marrow biopsies, cytogenetic, and next-generation sequencing (NGS) were obtained at the treating physician's discretion. Pathogenic/likely pathogenic variants (PV) were called based on the standard criteria. Overall survival (OS) was calculated from the time of t-MN diagnosis until the last follow up using Kaplan-Meier analysis using Wilcoxon test. For survival analysis comparing chemotherapy to best supportive care (BSC) only, patients were censored at the time of allogeneic stem cell transplant (SCT). Multivariate analysis was performed using Cox proportional hazard method and corrected using false discovery rate (FDR). Statistical analysis was performed using JMP (v14.1, SAS Institute) and significance was defined as P<0.05.
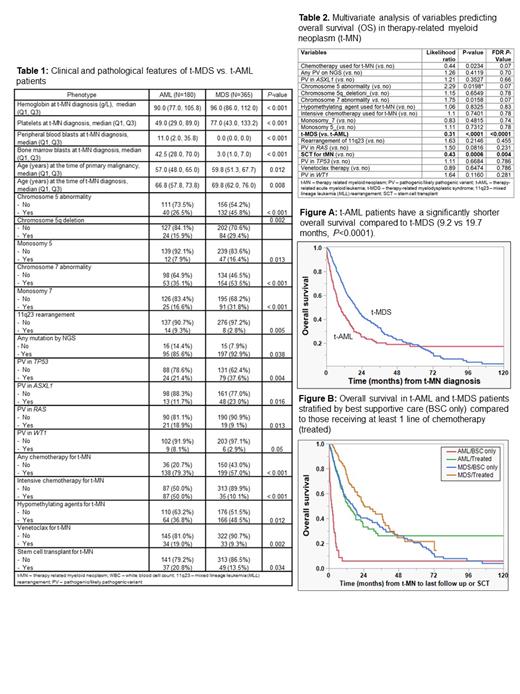
Results: We identified 554 patients, of which 180 (32.4%), 365 (65.8%), and 9 (1.6%) presented as t-AML, t-MDS, and t-MDS/MPN respectively. t-MDS/MPN patients were excluded from further analysis due to a small number. Clinical and laboratory characteristics of the t-AML and t-MDS cohorts is shown in Table 1. t-AML patients were significantly more anemic and thrombocytopenic at presentation. As expected, t-AML had a higher peripheral blood and BM blast count. There was no difference in proportion of patients with chromosomal abnormalities, though a statistically significantly higher proportion of t-MDS patients had chromosome 5 abnormality, 5q deletion, monosomy 5, chromosome 7 abnormality, monosomy 7 compared to t-AML patients. On other hand, 11q23 (mixed lineage leukemia, MLL) rearrangement was more common in t-AML compared to t-MDS (9.3% vs. 2.8%, P=0.005).
A higher proportion of t-MDS patients had PV detected by NGS compared to t-AML (92.9% vs. 85.6%, P=0.038). A higher proportion of t-MDS patients had PV in TP53 (37.6% vs. 21.4%, P=0.004) and ASXL1 (23% vs. 11.7%, P=0.016) genes; whereas a higher proportion of t-AML patients had PV in RAS (18.9% vs. 9.1%, P=0.013) and WT1 (8.1% vs. 2.9%, P=0.05) genes.
One hundred twenty-eight (35%) of 365 t-MDS patients progressed to t-AML. The presence of any chromosomal abnormality at t-MDS diagnosis predicted a higher risk of transformation to t-AML (χ 2 3.9, P=0.03).
t-AML patients had a significantly shorter OS compared to t-MDS (9.2 vs. 19.7 months, P<0.0001, Figure A). This difference persisted when stratified by no disease modifying therapies (BSC only) 2 vs. 17 months (P<0.0001, Figure B), as well as among those who received at least one line of chemotherapy (14 vs. 24.6 months, P<0.001). Finally, a higher proportion of patients with t-AML underwent SCT and there was a trend towards improved survival for t-AML patients (vs. t-MDS 52.9 vs. 20.7 months, P=0.07) from the time of transplant.
Multivariate analysis for OS performed to control for all the variables that were different at presentation (except for blast count), showed that t-MDS (as opposed to t-AML) phenotype at diagnosis, and undergoing SCT were independent predictors of improved survival (Table 2).
Conclusion: t-MDS and t-AML have distinct clinical, cytogenetic, and genetic features at presentation. In the absence of disease modifying therapies, t-AML is a more aggressive phenotype, consistently associated with a shorter survival. Even after controlling adverse risk features, t-AML phenotype had a shorter survival compared to t-MDS.
Litzow: AbbVie: Research Funding; Astellas: Research Funding; Amgen: Research Funding; Actinium: Research Funding; Pluristem: Research Funding; Jazz: Other: Advisory Board; Omeros: Other: Advisory Board; Biosight: Other: Data monitoring committee. Hiwase: Novartis: Membership on an entity's Board of Directors or advisory committees; AbbVie: Membership on an entity's Board of Directors or advisory committees.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal